New APEC Survey: More Regulatory Approval of Medical Products

Regulatory requirements for the approval of medical products are becoming more aligned for APEC economies, according to a new study by the APEC Life Sciences Innovation Forum (LSIF).
The survey, released on the sidelines of the 3rd APEC Senior Officials’ Meetings in host economy Chile, is APEC’s first foray into measuring progress in regulatory convergence in the medical sector. Regulatory convergence entails the adoption by authorities of internationally recognized standards and common or similar practices and procedures.
In 2009, APEC Ministers pledged to achieve convergence on approval procedures for medical products by 2020, to improve public access to life-saving innovations.
“Measurement leads to improvement. More regulatory convergence enhances the well-being of our people who rely on safe and effective medical products – and we look forward to achieving more progress,” said Dr. Michelle Limoli of the U.S. Food and Drug Administration, who also serves as co-chair of the forum’s Regulatory Harmonization Steering Committee (RHSC).
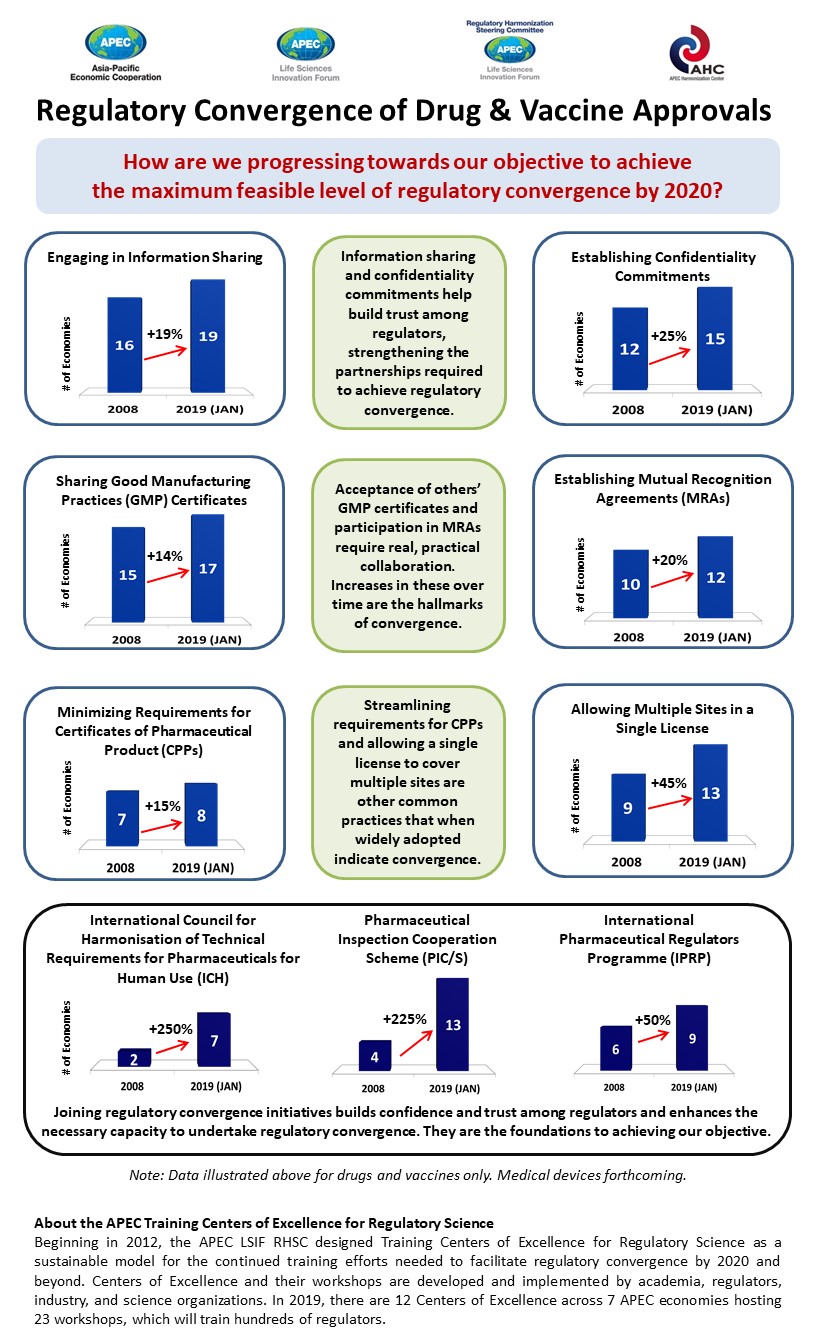
The survey results show a marked improvement in regulatory harmonization across APEC. In the last decade, all nine of the key performance indicators observed have improved significantly.
For example, the number of economies that have established mutual recognition agreements (MRAs), which allow regulators to recognize inspections conducted by foreign authorities, has increased by 20 percent. Now, over half of APEC economies have these MRAs in place.
The number of APEC economies recognizing good manufacturing practices certificates has increased by 14 per cent, which means far fewer duplication of inspection efforts in almost all APEC economies.
“When regulators take advantage of the testing, inspections, and reviews already completed by other regulators, they save public resources while delivering quality medical products,” said Dr. Nobumasa Nakashima of the Pharmaceuticals and Medical Devices Agency of Japan and co-chair of the RHSC.
Other gains include the increasing number of economies sharing information – to 19 of 21 economies – and more economies, 15 today, agreeing to confidentiality commitments. More economies are also streamlining requirements for certificates of pharmaceutical products and allowing a single license to cover multiple sites.
“We will continue tracking these indicators of regulatory convergence to show that economies are cooperating for the public good,” said Dr Dong-hee Lee, Director of the APEC Harmonization Center.
The RHSC also offers training centers that help regulators to understand and phase in the technical guidance and standards for the trade facilitation of medical products.
Today the 12 training centers located across seven APEC economies regularly train hundreds of regulators who study, amongst others, multi-regional clinical trials, biotechnological products, global supply chain integrity, advanced therapies, and medical devices. Participation in training boosts the credentials of authorities when they join global initiatives such as the International Pharmaceutical Regulators Forum and the International Medical Device Regulators Forum.

# # #
For further details, please contact:
Dini Sari Djalal +65 9137 3886 at [email protected]
Michael Chapnick +65 9647 4847 at [email protected]
More on APEC meetings, events, projects and publications can be found on www.apec.org. You can also follow APEC on Twitter and join us on Facebook, LinkedIn